Process & Test Method Validations
Whichever acronyms you know them by, we can help you with all your needs for Process Validation and Test Method Validation.
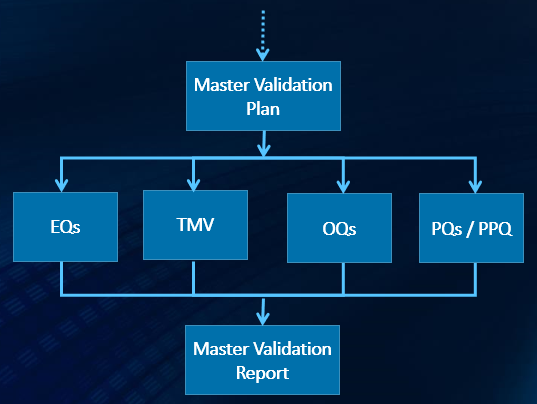
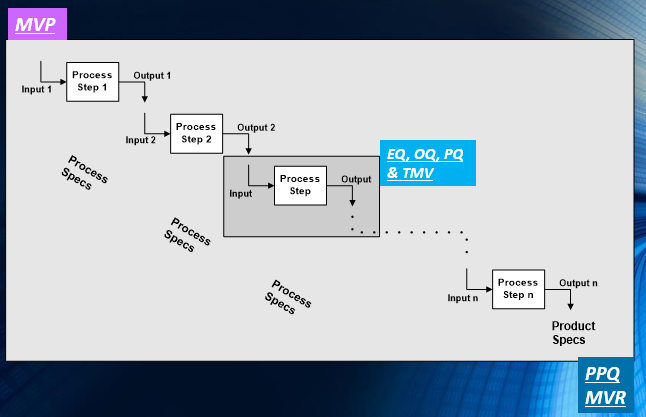
We know Process Validation so well that we have even developed a software app, InfoPV, to help you manage all of its activities and records.

